CAR T-cell Therapy Market Research Report Product (Abecma, Breyanzi), By Disease Indication (Lymphoma, Leukemia, Multiple Myeloma), By End-use, and by Region- Global Forecast to 2034
May-2025 Formats | PDF | Category: Healthcare | Delivery: 24 to 72 Hours
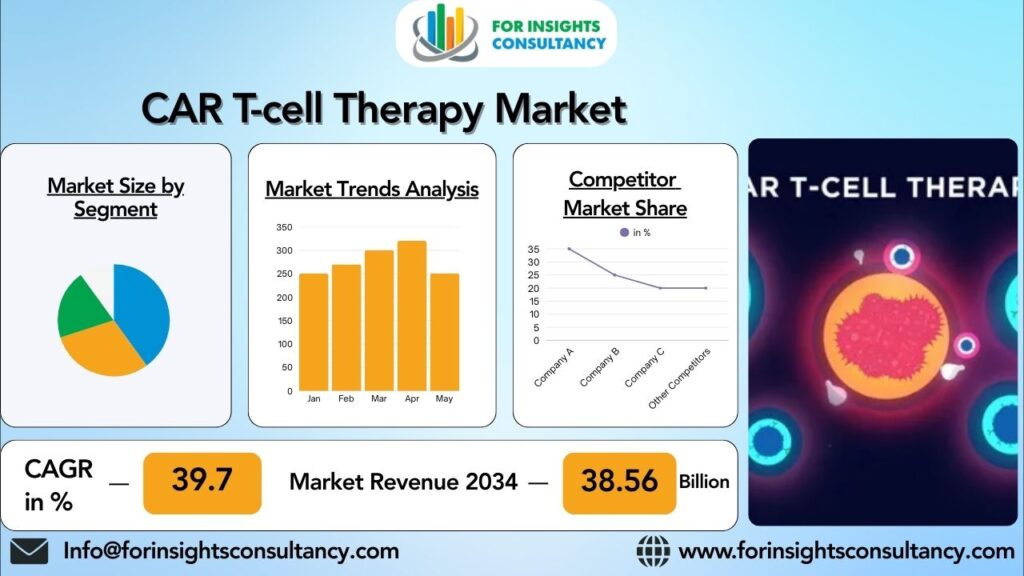
“The CAR T-cell Therapy Market industry is expected to expand from 6.3 Billion in 2025 to 38.56 Billion in 2034, with a compound annual growth rate of 39.7% “
CAR T-cell Therapy Market: Overview and Growth in the Upcoming Year
The field of cancer treatment has advanced significantly in recent years thanks to the introduction of innovative therapies like CAR T-cell therapy. This innovative technique uses the body’s immune system to target and destroy cancer cells. As the market for CAR T-cell treatment continues to grow, it is essential to comprehend the current state of affairs as well as the expected rise in the upcoming year.
CAR T-cell therapy involves reprogramming a patient’s T-cells to express chimeric antigen receptors (CARs) on their surface. These CARs specifically recognise and bind to the proteins on cancer cells, triggering an immune response that kills the cancerous cells. This tailored approach has shown promising results in the treatment of certain hematologic malignancies, such as leukaemia and lymphoma.
Due to increased investment in research and development, growing immunotherapy applications, and rising cancer prevalence, the market for CAR T-cell treatment has expanded quickly in recent years. As more clinical trials demonstrate the efficacy of CAR T-cell therapy, investors and pharmaceutical companies are growing more interested in this treatment.
Notwithstanding the promising potential of CAR T-cell therapy, problems such as exorbitant treatment expenses, challenging production, and potential adverse effects like cytokine release syndrome still need to be addressed. However, ongoing research and technological advancements are gradually improving the safety and effectiveness of CAR T-cell therapies, providing opportunities for market growth.
Patients now have access to these innovative medications thanks to the FDA and EMA licencing several CAR T-cell therapies for commercial use. The widespread application of CAR T-cell therapy is, however, hampered in some regions by barriers to market access and reimbursement laws. Collaboration between lawmakers and industry stakeholders is essential to resolving these issues and ensuring that patients have access to these life-saving treatments.
For Insights Consultancy’s latest market intelligence study, “Global CAR T-cell Therapy Market 2025, Growth Opportunities, and Forecast,” provides a comprehensive analysis of the Food industry. The report includes demand analysis, industry insights, competition intelligence, and a customer database. It also offers strategic insights into future trends, growth determinants, supplier landscape, demand landscape, CAGR, and pricing analysis. The study also includes Porter’s Five Forces Analysis, PESTLE Analysis, Value Chain Analysis, 4 Ps’ Analysis, Market Attractiveness Analysis, BPS Analysis, and Ecosystem Analysis.
*Note: Sample of the report provides details on the scope and coverage, table of contents, research methodology, and Sample Framework of the report. Actual report of 110+ is available for purchase to all the interested stakeholders.
Top Companies Covered In This Report
- Bristol-Myers Squibb Company
- Novartis AG
- Gilead Sciences, Inc.
- Johnson & Johnson Services, Inc.
- JW Therapeutics (Shanghai) Co., Ltd.
- bluebird bio, Inc.
- Merck & Co., Inc.
- Sangamo Therapeutics
- Sorrento Therapeutics, Inc.
- GSK plc.
Industry News
May 15, 2024 Bristol Myers Squibb’s CAR T Cell Therapy Breyanzi Approved by the U.S. Food and Drug Administration for Relapsed or Refractory Follicular Lymphoma
Bristol Myers Squibb announced the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Breyanzi ® (lisocabtagene maraleucel; liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) who have received two or more prior lines of systemic therapy. This indication is approved under accelerated approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). Breyanzi is also now included in the National Comprehensive Cancer Network (NCCN®) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-cell Lymphomas as a Category 2A recommendation for third-line and subsequent therapy for relapsed or refractory FL.*
29 October 2024 GSK enters agreement to acquire CMG1A46 from Chimagen Biosciences to expand immunology pipeline
GSK plc and Chimagen Biosciences (Chimagen), a privately held biotechnology company, today announced an agreement for GSK to acquire CMG1A46, a clinical-stage dual CD19 and CD20-targeted T cell-engager (TCE), from Chimagen for $300 million upfront. GSK plans to develop and commercialise CMG1A46 with a focus on B cell-driven autoimmune diseases, such as systemic lupus erythematosus (SLE) and lupus nephritis (LN), with potential to expand into related autoimmune diseases.
Detailed Segmentation and Classification of the report (Market Size and Forecast – 2034, Y-o-Y growth rate, and CAGR):
Segment By Product
- Abecma (idecabtagene vicleucel)
- Breyanzi (lisocabtagene maraleucel)
- Carvykti (ciltacabtagene autoleucel)
- Kymriah (tisagenlecleucel)
- Tecartus (brexucabtagene autoleucel)
- Yescarta (axicabtagene ciloleucel)
- Others
Segment By Disease
- Leukemia
- Lymphoma
- Multiple Myeloma
- Others
Segment By End-User
- Hospitals
- Cancer Treatment Centers
Regional Deep-dive Analysis:
The report provides in-depth qualitative and quantitative data on the CAR T-cell Therapy Market for all of the regions and countries listed below:
North America includes the United States, Canada, and Mexico.
Europe includes Germany, France, Italy, the United Kingdom, Scandinavia, Benelux, Russia, and the rest of Europe.
Asia-Pacific includes Japan, South Korea, India, China, Southeast Asia, and Australia.
South America (including Brazil, Argentina, and the rest of South America)
Middle East and Africa (Saudi Arabia, UAE, Israel, South Africa)
Each country is studied in detail, and the study includes qualitative and quantitative analysis of the CAR T-cell Therapy Market in that country.
North America
CAR T-cell therapy has become a leading treatment option for a number of tumours in North America, especially in the US. Leading pharmaceutical businesses and research institutions in the area are spearheading improvements in this discipline with a strong emphasis on research and development. The market for CAR T-cell treatment has expanded in North America due to regulatory approvals and rising clinical trial investments.
Europe
The use of CAR T-cell therapy as a cancer treatment has been pioneered in Europe by countries such as France, Germany, and the United Kingdom. Because precision oncology and personalised medicine are becoming more and more important, CAR T-cell therapy is gradually being incorporated into standard treatment procedures in European healthcare systems. This sector offers a profitable market for CAR T-cell therapy manufacturers and innovators.
Asia-Pacific
Asia Pacific countries like China, Japan, and South Korea are seeing an increase in demand for advanced cancer treatments like CAR T-cell therapy. Thanks to government initiatives that support biotechnology and oncology research and development, the market for CAR T-cell treatment is growing in a favourable environment. Strategic partnerships and collaboration with global biopharmaceutical companies are driving advancements in this field.
Middle East and Africa
The Middle Eastern countries of Israel, Saudi Arabia, and the United Arab Emirates are anticipated to become significant players in the CAR T-cell therapy market. Due to the increased focus on building out healthcare infrastructure and investing in cutting-edge medical technologies, the Middle East offers significant opportunities for CAR T-cell treatment providers. Clinical studies and collaborations with regional healthcare providers are promoting the adoption of this innovative therapeutic approach.
The research provides answers to the following key questions:
- What is the expected growth rate of the CAR T-cell Therapy Market from 2025-2034?
- What are the key driving forces shaping the market during the forecast period?
- Who are the major market vendors and what winning strategies have helped them occupy a strong foothold in the CAR T-cell Therapy Market?
- What are the prominent market trends influencing the market’s development?
Key insights provided by the report that could help you take critical strategic decisions?
- Regional reports analyse product/service consumption and market factors in each region.
- Reports highlight possibilities and dangers for suppliers in the CAR T-cell Therapy Market business globally.
- The report identifies regions and sectors with the highest growth potential.
- It provides a competitive market ranking of major companies, as well as information on new product launches, partnerships, business expansions, and acquisitions.
- The report includes a comprehensive corporate profile with company overviews, insights, product benchmarks, and SWOT analysis for key market participants.
Customization: We can provide following things
1) On request more company profiles (competitors)
2) Data about particular country or region
3) We will incorporate the same with no additional cost (Post conducting feasibility).
Any Requirement Contact us: https://forinsightsconsultancy.com/contact-us/
Table of Contents
For TOC Contact us: https://forinsightsconsultancy.com/contact-us/